Tribovax 10 is a 4 in 1 clostridial vaccine for cattle and sheep for Blackleg, Braxy, Black Disease and Tetanus.
Volume:
This product is only licensed for sale in the Republic Of Ireland
Tribovax 10 is a 4 in 1 clostridial vaccine for cattle and sheep for Blackleg, Braxy, Black Disease and Tetanus.
Vaccination with Tribovax 10 induces an active immunity that protects the vaccinated animal for a period of up to 12 months.
The long duration of immunity of Tribovax 10 ensures that vaccinated animals are protected all the year round and simplifies management procedures on the farm.
Lambs and calves can be vaccinated from as young as 2 weeks of age.
Vaccinating pregnant animals during the 2-6 weeks prior to parturition transfers immunity via the colostrum to the newborn animals shortly after birth and will protect the young animals for a period of 8-12 weeks.
Tribovax 10 has been proven efficacious in the presence of maternally derived antibodies.
It is at 8-12 weeks old recommended that animals with unknown colostrum status be vaccinated from 2 weeks of age.
Those animals from vaccinated mothers and with confirmed colostrum intake should be vaccinated
Active substances
C. perfringens type A (α) toxoid ≥ 0.5 IU#
C. perfringens type B & C (β) toxoid ≥ 18.2 IU*
C. perfringens type D (ε) toxoid ≥ 5.3 IU*
C. chauvoei whole culture, inactivated ≥ 90% protection**
C. novyi toxoid ≥ 3.8 IU*
C.septicum toxoid ≥ 4.6 IU*
C. tetani toxoid ≥ 4.9 IU*
C. sordellii toxoid ≥ 4.4 U1
C. haemolyticum toxoid ≥ 17.4 U#
Target Species
Cattle and Sheep
Treats and Controls
Disease associated with infections caused by Clostridium perfringens types
Application Method
Subcutaneous Injection
Withdrawal Period
Zero Days
Dosage
Dose:
- Sheep: 1 ml – from 2 weeks of age
- Cattle: 2 ml – from 2 weeks of age
The primary course of immunisation consists of two injections, allowing an interval of four to six weeks between them.
The course should be completed at least 2 weeks before maximum immunity is required.
Minimum age 3 months.
Booster vaccination is required at 6 monthly intervals for continuous protection but where there is no period of risk in winter annual booster vaccination is all that is necessary.
Once opened use of the vaccine must be completed the same day
Key Features of Tribovax 10
This Product is only licensed for sale in the Republic of Ireland
Click here to Download Data Sheet
Health Products Regulatory Authority
Summary of Product Characteristics
1 NAME OF THE VETERINARY MEDICINAL PRODUCT
Tribovax 10 suspension for injection for cattle and sheep
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each 1 ml of vaccine contains:
Active substances
C. perfringens type A (α) toxoid ≥ 0.5 IU#
C. perfringens type B & C (β) toxoid ≥ 18.2 IU*
C. perfringens type D (ε) toxoid ≥ 5.3 IU*
C. chauvoei whole culture, inactivated ≥ 90% protection**
C. novyi toxoid ≥ 3.8 IU*
C.septicum toxoid ≥ 4.6 IU*
C. tetani toxoid ≥ 4.9 IU*
C. sordellii toxoid ≥ 4.4 U1
C. haemolyticum toxoid ≥ 17.4 U#
* ELISA According to Ph.Eur.
1 In house ELISA
** Guinea pig challenge test according to Ph.Eur.
# In vitro toxin neutralisation test based on haemolysis of sheep erythrocytes.
Adjuvant
Aluminium1 3.026 – 4.094 mg
1 from aluminium potassium sulphate (alum)
Excipient
Thiomersal 0.05 – 0.18mg
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Suspension for injection
Light brown aqueous suspension that settles on storage.
4 CLINICAL PARTICULARS
4.1 Target Species
Cattle and sheep.
4.2 Indications for use, specifying the target species
For the active immunisation of sheep and cattle against disease associated with infections caused by Clostridium perfringens type A, C. perfringens type B, C. perfringens type C, C.perfringens type D, Clostridium chauvoei, Clostridium novyi type B, Clostridium septicum, Clostridium sordellii and Clostridium haemolyticum and against tetanus caused by Clostridium tetani.
For the passive immunisation of lambs and calves against infections caused by the above mentioned clostridial species (except C. haemolyticum in sheep).
Onset of immunity:
Sheep and Cattle: Two weeks after the basic vaccination course (as demonstrated by serology only).
Duration of active immunity:
As demonstrated by serology only:
Sheep: 12 months against C. perfringens type A, B, C and D, C. novyi type B, C. sordellii, C. tetani < 6 months against C. septicum, C. haemolyticum, C. chauvoei
Cattle: 12 months against C. tetani and C. perfringens type D < 12 months against C. perfringens type A, B and C < 6 months against C. novyi type B, C. septicum, C. sordellii, C. haemolyticum, C. chauvoei
An anamnestic humoral immune response (immunological memory) to all components was demonstrated 12 months following the basic course of vaccination.
Duration of passive immunity:
As demonstrated by serology only:
Lambs:
At least 2 weeks for C. septicum and C. chauvoei At least 8 weeks for C. perfringens type B and C. perfringens type C
At least 12 weeks for C. perfringens type A, C. perfringens type D, C. novyi type B, C. tetani and C. sordellii
No passive immunity was observed for C. haemolyticum.
Calves:
At least 2 weeks for C. sordellii and C. haemolyticum At least 8 weeks for C. septicum and C. chauvoei
At least 12 weeks for C. perfringens type A, C. perfringens type B, C. perfringens type C, C. perfringens type D, C. novyi type B, and C. tetani
4.3 Contraindications
Do not use in sick or immunodeficient animals.
4.4 Special warnings for each target species
Vaccinate healthy animals only.
The effectiveness of the vaccine in providing passive immunity to young lambs and calves depends on these animals ingesting adequate amounts of colostrum on the first day of life.
Clinical trials have demonstrated that the presence of maternal derived antibodies (MDA), particularly against C. tetani, C. novyi type B, C. perfringens type A (calves only), C. chauvoei (lambs only) and C. perfringens type D may reduce the antibody response to vaccination in young lambs and calves.
Therefore, to ensure an optimal response in young animals with high levels of MDA, the basic vaccination should be delayed until the levels wane (which is after about 8-12 weeks of age, see section 4.2).
4.5 Special precautions for use
Special precautions for use in animals
It is good practice to observe animals regularly for adverse reactions at the injection site following vaccination.
It is recommended to seek medical advice from a veterinarian in case of a severe injection site reaction.
Special precautions to be taken by the person administering the veterinary medicinal product to animals
In case of accidental self-injection, seek medical advice immediately and show the package leaflet or the label to the physician.
4.6 Adverse reactions (frequency and seriousness)
Swelling at the injection site was observed very commonly in clinical studies.
This may reach up to a mean value of 6 cm in sheep and 15 cm diameter in cattle; sometimes reactions of up to 25 cm diameter may be seen in cattle.
Most local reactions resolve within 3-6 weeks in sheep and in less than 10 weeks in cattle. In a minority of animals they may persist longer.
An abscess may develop commonly.
Skin discolouration at the injection site (which returns to normal as the local reaction resolves) may occur commonly. Mild hyperthermia may occur commonly.
Localised pain at the injection site for 1-2 days post first vaccination may occur uncommonly.
Anaphylactic reactions were observed in very rare cases in spontaneous pharmacovigilance reports.
In such cases appropriate treatment such as adrenaline should be administered without delay.
The frequency of adverse reactions is defined using the following convention:
- very common (more than 1 in 10 animals treated displaying adverse reaction(s))
- common (more than 1 but less than 10 animals in 100 animals treated)
- uncommon (more than 1 but less than 10 animals in 1,000 animals treated)
- rare (more than 1 but less than 10 animals in 10,000 animals treated)
- very rare (less than 1 animal in 10,000 animals treated, including isolated reports).
4.7 Use during pregnancy, lactation or lay
Pregnancy:
No side effects other than those described under section 4.6 were seen when the vaccine was used in sheep and cattle between 8 and 2 weeks prior to parturition.
In the absence of specific data, the use of the vaccine is not recommended during the first or second third of pregnancy.
4.8 Interaction with other medicinal products and other forms of interactions
No information is available on the safety and efficacy of this vaccine when used with any other veterinary medicinal product.
A decision to use this vaccine before or after any other veterinary medicinal product therefore needs to be made on a case by case basis.
4.9 Amounts to be administered and administration route
Subcutaneous use.
Dose:
- Sheep: 1 ml – from 2 weeks of age
- Cattle: 2 ml – from 2 weeks of age
Administration:
By subcutaneous injection preferably in the loose skin on the side of the neck, observing aseptic precautions.
Shake the bottle thoroughly before use.
Syringes and needles should be sterile before use and the injection should be made through an area of clean, dry skin taking precautions against contamination.
Basic vaccination:
Two doses should be administered, 4-6 weeks apart (see section 4.2 and 4.4).
Re-vaccination:
A single dose should be administered at 6 to 12 month intervals after the basic vaccination (see section 4.2.)
Use in pregnancy:
To provide passive protection of the offspring, via the colostrum, a single re-vaccination should be administered between 8 and 2 weeks before parturition, provided that animals have received a full basic vaccination course before pregnancy.
4.10 Overdose (symptoms, emergency procedures, antidotes), if necessary
In calves and lambs, local reactions may increase slightly if twice the recommended dose is administered (see section 4.6).
4.11 Withdrawal period(s)
Zero days.
5 PHARMACOLOGICAL or IMMUNOLOGICAL PROPERTIES
Pharmacotherapeutic group:
Immunologicals for Bovidae and Ovidae,
inactivated bacterial vaccines (including mycoplasma, toxoid and chlamydia) for cattle and sheep, clostridium. ATC-vet code: QI02AB01, QI04AB01.
Inactivated clostridium vaccine.
To stimulate active immunity in sheep and cattle against C. chauvoei and the toxins of Clostridium perfringens type A, C. perfringens type B, C. perfringens type C, C. perfringens type D, C. novyi, C. septicum, C. tetani, C. sordellii, and C. haemolyticum contained in the vaccine.
To provide passive immunity via the colostrum against the above mentioned clostridial infections in young lambs and calves.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Aluminium potassium sulphate (alum)
Thiomersal Sodium chloride
Water for injections
Formaldehyde
6.2 Major incompatibilities
Do not mix with any other veterinary medicinal product.
6.3 Shelf-life
Shelf-life of the veterinary medicinal product as packaged for sale: 30 months.
Shelf-life after first opening the immediate packaging: 8 hours.
6.4 Special precautions for storage
Store and transport refrigerated (2 °C - 8 °C).
Do not freeze.
Protect from light.
6.5 Nature and composition of immediate packaging
Flexible low density polyethylene (LDPE) bottle with 20 ml, 50 ml, or 100 ml, closed with a bromobutyl rubber stopper and held in place with an aluminium cap.
Pack sizes: Cardboard box with one bottle of 20 ml (20 doses of 1 ml or 10 doses of 2 ml).
Cardboard box with one bottle of 50 ml (50 doses of 1 ml or 25 doses of 2 ml).
Cardboard box with one bottle of 100 ml (100 doses of 1 ml or 50 doses of 2 ml).
Not all pack sizes may be marketed.
6.6 Special precautions for the disposal of unused veterinary medicinal products or waste materials derived from the use of such products
Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal products should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
Intervet Ireland Limited
Magna Drive
Magna Business Park,
Citywest Road
Dublin 24
Ireland
8 MARKETING AUTHORISATION NUMBER(S)
VPA10996/286/001
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 10 September 2021
10 DATE OF REVISION OF THE TEXT
January 2022
Cattle Injectables
Injectables should be given according to the manufacturer’s instructions at the recommended injection site.
• Always use a clean, sterile syringe and needle. If using a multiple injection gun, ensure the needle is disinfected between injections, e.g. with an automatic sterilisation system.
• If the site to be injected is dirty, clean the skin and swab with an alcohol-impregnated wipe or cotton wool.
• Before injecting, check the expiry date and read the instructions of the product to be used. Some products need to be shaken before use.
• Use the correct-sized needle according to the size of the animal and site of injection.
• Ensure the animal is adequately restrained before attempting the injection.
• Take care to ensure it is given subcutaneously and not intramuscularly. Raise a fold of skin at the injection site (mainly neck but some are ear) recommended by the product manufacturer and inject carefully into the space created.
• If a large dose is to be delivered, it may be advisable to split the dose between two injection sites. After the injection, briefly massage the site to improve the dispersal of the injected material.
• Dispose of the needle and syringe in appropriate clinical waste and sharps containers.
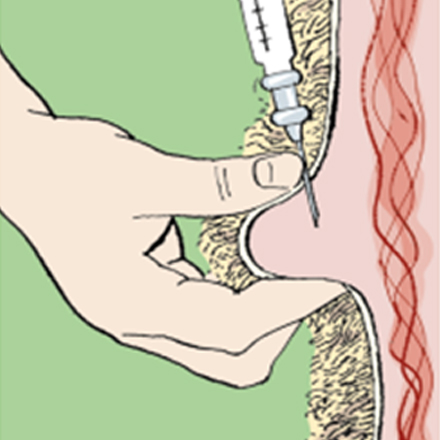
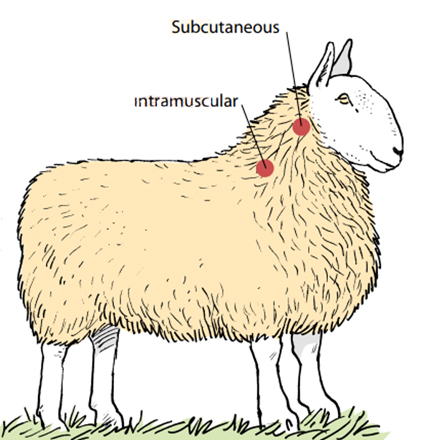
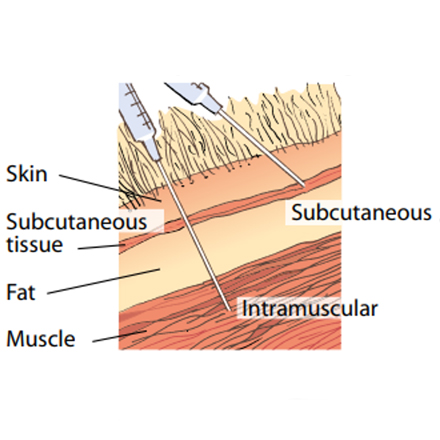
Sheep Subcutaneous injections
Subcutaneous injections need to be administered with care to ensure the product is placed under the skin and not into the fleece or muscle.
The sheep needs to be well restrained, and the skin ‘tented’ away from the underlying muscle.
The preferred injection site is 10–15 cm (4–6 inches) below the ear on the side of the neck (see diagram below). Usually a 1.6 cm (5/8 inch) needle is ideal.
After administration, the site should be gently massaged.
Here at Agridirect we have joined forces with Parcelforce to ensure all packages are delivered promptly and safely to you. We ship to all ares within mainland United Kingdom and Northern Ireland. Deliveries take place Monday to Friday excluding bank holidays. Once your order has been dispatched from our warehouse you will be notified by email. If there is a delay with your order for any reason you will be contacted immediately. Orders take 2-4 Working days to be delivered. Some products have an extended delivery time, this is noted on the individual products.
| Country | Orders Under £100 | Orders Over £100 |
| United Kingdom (Mainland Only) | £5.00 | £0.00 |
| Northern Ireland | £5.00 | £0.00 |
There may be an addition charge on certain bulky items. This charge will be clearly marked on an applicable products and will be explained on the checkout page before payment has been made.
We’re sorry your purchase didn’t work out. But don’t worry; we have a great returns policy to help you out.
All purchases can be returned to us within 14 days of delivery and returned goods must be received within 14 days from the date you informed us of the return.
Items returned during the cooling-off period must be in a resalable condition, which means:
If we discover goods have been used or there has been a loss in value of the goods due to damage to the goods or the packaging, while in your care or whilst being returned to us, we will reduce the amount refunded, which may amount to the full cost of the product, to cover loss of value of goods.
All returns should be complete which includes boxes, manuals and accessories that may have been included with the order.
All returns must be packaged appropriately for shipping, we will not accept responsibility for damages or loss which occur during shipping of a return product.
We accept no responsibility for goods damaged or lost while in transit to us.
The cost of the return, including all packaging and freight, lies solely with the consumer.
First off, if you have received a damaged electrical product from us, do not plug it in. Any electrical products that are plugged in are deemed ‘as used and accepted’ and are not accepted as returns. All damages must be reported to us via phone or email within 24hours of receipt of goods. Please ensure you check your items upon delivery.
How do I begin the returns process?
If you wish to begin the return process, please email us at sales@agridirect.co.uk and ensure the following information is included in your email. Your name, phone number, Order id, the item you wish to return, reason for return and if the product is damaged we require photos of the product.
Once you have sent us all required information a member of our team will assess your claim and will contact you as soon as possible. Please hold off on returning products until a member of our team has called you to confirm.
All returns must be accompanied with a fully filled out returns form which can be downloaded here.
All returns must be the complete product which includes boxes, manuals and accessories that may have been include with the order.
All returns must be packaged appropriately for shipping, we will not accept responsibility for damages or loss which occur during shipping of a return product.
Address: Please ship the product to the following address: Agridirect UK, C/O Parcel Club, 6 Derrintony Road, Derrylin, Co Fermanagh, BT92 9EZ
Once the returned product has been returned to us and fully inspected a refund will be issued.
Please Note: A typical timeline for a refund to show in your account is up to 10 working days from the date processed, depending on your bank.
Would you like to send this voucher to the recipient via email?
Yes No