Volume:
Fasinex 240 is an oral drench for cattle that is used for the treatment and control of all three stages of liver fluke, mature, immature and early immature. Fasinex 240 is a concentrated, low volume drench that is easy to administer.
Active Ingredient: Triclabendazole
Target Species: Cattle
Administration Method: Oral Drench
Treats and Controls: Mature, immature and early immature liver fluke
Withdrawal Time: 56 days for animals intended for meat and offal. For dairy cattle milk for human consumption may only be taken from 48 hours after calving. If calving occurs before 35 days after treatment, milk for human consumption may only be taken after 35 days plus 48 hours after the treatment.
Dosage: 2.5 ml per 50 kg of bodyweight
| Body Weight | Dose Volume | Number of full doses per pack: | |
| 2.2 Litre | 5 Liter | ||
| 50kg | 2.5 ml | 880 | 2000 |
| 100kg | 5 ml | 440 | 1000 |
| 150kg | 7.5 ml | 293 | 667 |
| 200kg | 10 ml | 220 | 500 |
| 250kg | 12.5 ml | 176 | 400 |
| 300kg | 15 ml | 147 | 333 |
| 350kg | 17.5 ml | 126 | 286 |
| 400kg | 20 ml | 110 | 250 |
| 450kg | 22.5 ml | 98 | 222 |
| 500kg | 25 ml | 88 | 200 |
| 550kg | 27.5 ml | 80 | 182 |
| 600kg | 30 ml | 73 | 167 |
Always read the label and all enclosed information for Fasinex 240 before administering to animals!
How Livestock Contracts Liver Fluke
Animals are infected by ingesting encapsulated larvae (metacercariae) on contaminated grass. Typically,individual farms will have wet “flukey areas” that should not be used or grazed at times of the year when metacercariae are likely to be present, i.e. late summer to winter (depending on climatic conditions). However,cattle and sheep often graze on such areas. Therefore, it cannot be assumed that liver fluke infection is absent from any area of Ireland.

This product is only licensed for sale within the Republic of Ireland
Click here to Download Data Sheet
1 NAME OF THE VETERINARY MEDICINAL PRODUCT
Fasinex 240 mg/ml oral suspension for cattle.
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Active Substance:
Triclabendazole 240 mg
Excipients:
Methyl parahydroxybenzoate (E218) 1.1 mg
Propyl parahydroxybenzoate (E216) 0.4 mg
Benzyl alcohol (E1519) 5.0 mg
3 PHARMACEUTICAL FORM
Oral Suspension.
White to cream-coloured aqueous suspension.
4 CLINICAL PARTICULARS
4.1 Target Species
Cattle.
4.2 Indications for use, specifying the target species
For the treatment of acute, subacute and chronic infection due to early immature, immature, and mature stages of
Fasciola hepatica. If infected animals are treated before disease has developed, fasciolosis can be prevented.
4.3 Contraindications
Do not use in case of hypersensitivity to the active substance or to any of the ingredients.
4.4 Special warnings for each target species
Care should be taken to avoid the following practices because they increase the risk of development of resistance and could ultimately result in ineffective therapy.
• Too frequent and repeated use of anthelmintics from the same class, over an extended period of time.
• Underdosing, which may be due to underestimation of bodyweight, misadministration of the product, or lack
of calibration of the dosing device (if any).
Suspected clinical cases of resistance to anthelmintics should be further investigated using appropriate tests (e.g. Faecal Egg Count Reduction Test). Where the results of the test(s) strongly suggest resistance to a particular anthelmintic, an anthelmintic belonging to another pharmacological class and having a different mode of action should be used.
Resistance to triclabendazole has been reported in Fasciola hepatica in a number of countries including ones in the EU.
Therefore the use of this product should be based on local epidemiological information about susceptibility of F.
hepatica and recommendations on how to limit further selection for resistance to anthelmintics.
Dosing programmes should be discussed with your Veterinary Adviser.
4.5 Special precautions for use
i) Special precautions for use in animals
Only for use for liver fluke strains susceptible to triclabendazole.
Intensive use or misuse of anthelmintics can give rise to resistance.
To reduce the risk, dosing programs should be discussed with your veterinary practitioner.
Efficacy of this product against liver fluke is reduced if triclabendazole resistant strains are present.
Where a dosing gun is used to administer the product, care must be taken to avoid the occurrence of dosing gun
pharyngitis. Not intended for use within 35 days of calving.
ii) Special precautions to be taken by the person administering the veterinary medicinal product to animals
Do not eat, drink, or smoke while handling the product.
Wash hands and exposed skin after handling the product.
In case of accidental spillage onto skin or in eyes, wash immediately with water.
Take off any contaminated clothes.
iii) Other precautions
None known.
4.6 Adverse reactions (frequency and seriousness)
None known.
4.7 Use during pregnancy, lactation or lay
Fasinex is neither embryotoxic nor teratogenic, and is safe for use in all stages of pregnancy and lactation.
However, the product is not permitted for use in lactating animals producing milk for human consumption.
4.8 Interaction with other medicinal products and other forms of interaction
None known.
4.9 Amounts to be administered and administration route
To ensure administration of a correct dose, bodyweight should be determined as accurately as possible; accuracy of the dosing device should be checked.
If animals are to be treated collectively rather than individually they should be grouped according to their bodyweight and dosed accordingly, in order to avoid under- or over-dosing.
Administer 5 ml/100 kg body weight, equivalent to 12 mg triclabendazole per kg of body weight.
Fasinex is administered orally after thorough shaking of the suspension.
Most types of automatic drenching guns are suitable.
Clean drenching gun before and after use.
Fasinex can safely be given to young, pregnant or stressed cattle.
However, the product is not permitted for use in lactating animals producing milk for human consumption.
Fasinex is given once.
The administration may be repeated several weeks or months after the first treatment depending on the epidemiological situation.
In case of sub-acute and acute fasciolosis, affected cattle, usually young animals, should be treated immediately after diagnosis is reached.
Advice from your prescriber or veterinary surgeon should be sought for subsequent dosing intervals.
Shake container well before use.
Dosing Table
Add 5 ml for each additional 100 kg
Dosing recommendations:
On land where sheep are being treated according to a preventative programme and where cattle are also grazing these areas, Fasinex should be administered to the cattle on the same treatment dates as the sheep.
Fasinex 5% should be used in sheep.
Treatment times should be customised under veterinary advice for each individual farm.
Bought in animals:
All bought in animals should be dosed before joining the main herd unless there is evidence of triclabendazole
resistance in those cattle.
Housed cattle:
Dose cattle, which have grazed fluke infected pasture in the autumn at the time of or shortly after housing. Dosing may be required to be started at the beginning of the fluke season when animals are still outdoors depending on the specific farm situation.
Treatment of acute outbreaks:
The herd should be treated immediately after diagnosis and veterinary advice should be sought for subsequent dosing intervals.
BodyWeight (kg) Volume to Administer (ml)
Up to 50 kg 2.5
>50-70 3.5
>70-100 5
>100-150 7.5
>150-200 10
>200-300 15
>300-400 20
>400-500 25
4.10 Overdose (symptoms, emergency procedures, antidotes), if necessary
A single oral dose of 150-200 mg triclabendazole/kg of body weight (more than 12 times the recommended dose rate)
was shown to lead to side effects such as stumbling gait, depression, and decreased appetite.
These side effects are slight and last 1 to 3 days.
An antidote is not known.
4.11 Withdrawal Period(s)
Meat and offal: 56 days.
Milk: Milk for human consumption may only be taken from 48 hours after calving.
If calving occurs before 35 days after treatment, milk for human consumption may only be taken after 35 days plus 48 hours after the treatment.
5 PHARMACOLOGICAL or IMMUNOLOGICAL PROPERTIES
Pharmacotherapeutic group:
triclabendazole is a benzimidazole anthelmintic
ATCvet code:
QP52AC01
5.1 Pharmacodynamic properties
Triclabendazole inhibits cellular transport mechanisms and binds to a different tubulin receptor, possibly the tubulozole receptor, than do other benzimidazoles, which bind to the colchicine receptor. Triclabendazole also inhibits protein synthesis.
5.2 Pharmacokinetic properties
Triclabendazole is readily absorbed and oxidised to its sulfoxide and sulfone.
Triclabendazole sulfoxide reaches peak concentrations approximately 1 day after administration of Fasinex and the sulfone reaches peak concentrations approximately 3 days after administration.
Both metabolites bind strongly to plasma protein, particularly albumin.
Metabolites are excreted via the bile, primarily as conjugates.
More than 90% of the total dose of Fasinex is excreted in the faeces, about 5% in the urine and 1% in milk.
Elimination is virtually complete by 10 days after administration.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Methyl parahydroxybenzoate (E218)
Propyl parahydroxybenzoate (E216)
Benzyl alcohol (E1519)
Microcrystalline cellulose and carmellose sodium
Povidone
Simethicone emulsion
Propylene glycol
Purified water
6.2 Incompatibilities
None known.
6.3 Shelf-life
Shelf-life of the veterinary medicinal product as packaged for sale: 3 years.
Shelf-life after first opening the immediate packaging: 12 months.
6.4 Special precautions for storage
Store in tightly closed original container.
6.5 Nature and composition of immediate packaging
High density polyethylene bottles of 0.8, 2.2, 5.0, and 12.0 litres.
Not all pack sizes may be marketed.
6.6 Special precautions for the disposal of unused veterinary medicinal products or waste materials
Any unused veteinary medicinal product or waste materials derived from such veterinary medicinal product should be
disposed of in accordance with local requirements.
Do not contaminate ponds, waterways or ditches with the product or used container.
7 MARKETING AUTHORISATION HOLDER
Novartis Animal Health UK Limited
Frimley Business Park
Frimley
Camberley
Surrey GU16 7SR
United Kingdom
8 MARKETING AUTHORISATION NUMBER(S)
VPA: 10825/004/002
9 DATE OF THE FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 5th September 2008
Date of last renewal: 24th May 2013
10 DATE OF REVISION OF THE TEXT
February 2014
Drench correctly
Cattle Oral drenches
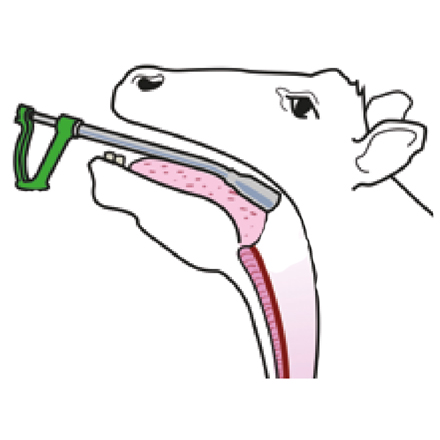
Oral drenching guns are designed to deliver the treatment towards the back of the mouth over the tongue, so the entire dose is swallowed at once to optimise efficacy.
• Make sure animals are properly restrained, with their head held up • Slide the nozzle of the dosing gun in the side of the mouth and over the tongue so that the entire dose is swallowed immediately
• Drenching equipment must be correctly calibrated and in good working order
• Calibrate the gun using the product just before treatment starts by delivering two or more doses into a graduated measuring cylinder Faulty equipment, or attempting to dose too quickly, may mean that the barrel of the gun does not fill properly or that the liquid is full of bubbles.
Dosing Weight – do not guess. Underestimating the weight of sheep is a common cause of underdosing. Select and weigh the biggest sheep in the group to determine the correct dose. If there is a wide range of weights, consider splitting the group, then weigh the heaviest in each section. Do not forget to check that the weigh crate is accurate before starting!
Calibrate and maintain the drench gun
Always check the gun is delivering the right amount before you drench. Remove the plunger from a 10 ml syringe, put a thumb over the end and squirt the dose into it, making sure there are no air bubbles left. Adjust the gun until the dose delivered is correct. Drenching guns should also be well maintained and replaced regularly. Clean with warm soapy water after use and check springs and tubes to make sure there are no kinks that will form air bubbles.
Withholding food
Research has shown that the efficacy of the white (BZ) and clear (AV) drenches can be improved by withholding food for 12–24 hours before treatment. It is not advised to deprive heavily pregnant ewes of food, so if you treat this class of stock with anthelmintics, you may wish to use yellow drenches (LV) because their efficacy is less dependent on rumen fill.
Storage
Wormers should be stored securely, away from direct sunlight at 4–25°C. Check the use-by date and, once open, use within the time shown on the packaging. Shake white (BZ) products well before use.
Pharmacodynamics
Triclabendazole and its metabolites are active against both the immature and mature worms of Fasciola hepatica and Fasciola gigantica helminths.
Mechanism of action
Triclabendazole is an anthelmintic agent against Fasciola species.
The mechanism of action against Fasciola species is not fully understood at this time.
In vitro studies and animal studies suggest that triclabendazole and its active metabolites (sulfoxide and sulfone) are absorbed by the outer body covering of the immature and mature worms, causing a reduction in the resting membrane potential, the inhibition of tubulin function as well as protein and enzyme synthesis necessary for survival. These metabolic disturbances lead to an inhibition of motility, disruption of the worm outer surface, in addition to the inhibition of spermatogenesis and egg/embryonic cells.
A note on resistance
In vitro studies, in vivo studies, as well as case reports suggest a possibility for the development of resistance to triclabendazole.
The mechanism of resistance may be multifactorial and include changes in drug uptake/efflux mechanisms, target molecules, and changes in drug metabolism.
Would you like to send this voucher to the recipient via email?
Yes No